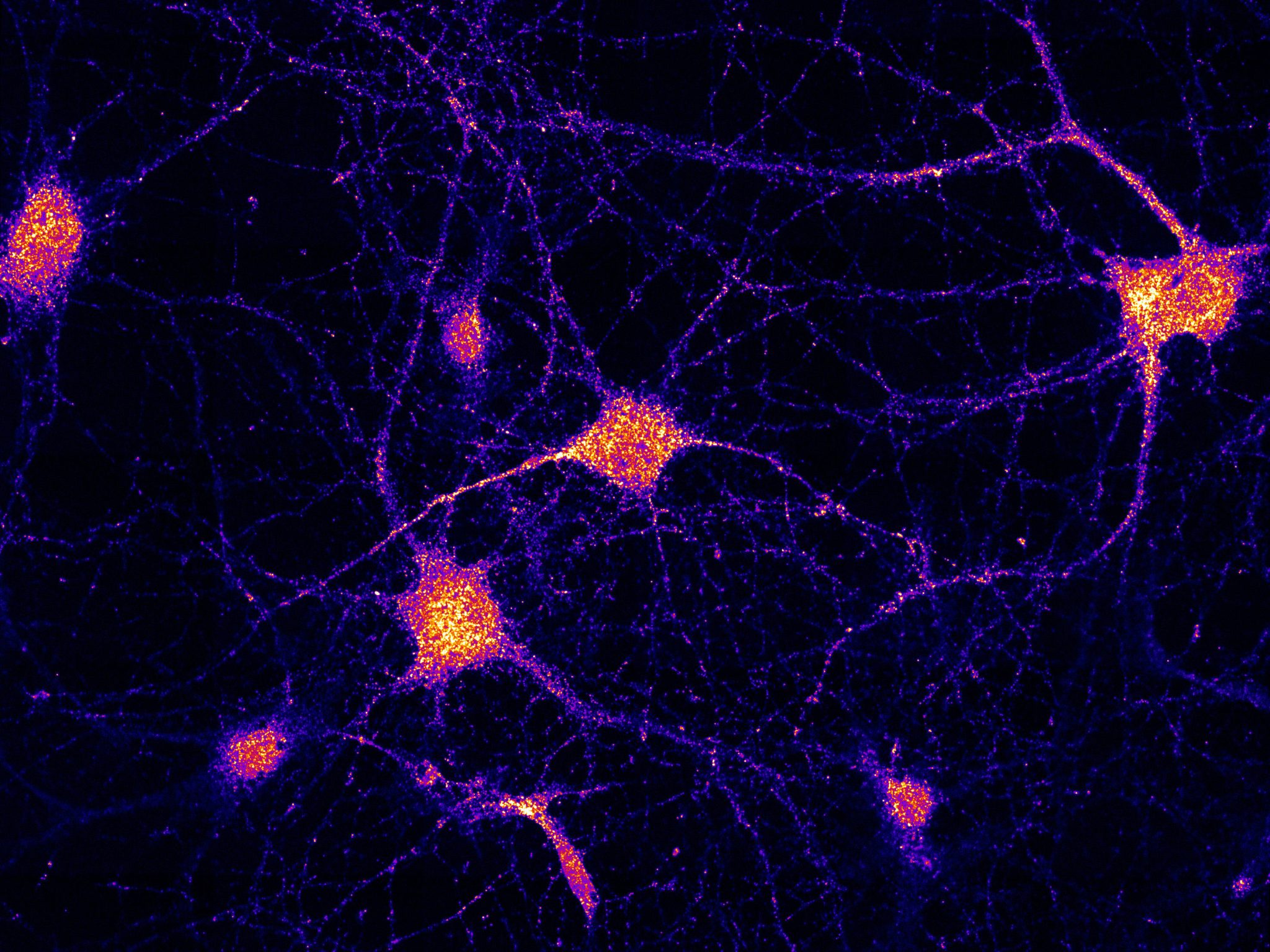
Le ferry peut voyager dans les deux sens le long des réseaux routiers à l’intérieur des cellules. Crédit : Schuhmacher et al. (2023) / MPI-CBG
Les efforts de collaboration des équipes des instituts MPI situés à Dresde, Dortmund, Francfort-sur-le-Main et Göttingen ont conduit à la première preuve d’un complexe protéique qui joue un rôle essentiel dans le transport des messagers.[{ » attribute= » »>RNA in neurons.
Every part of brain cells, even their lengthy offshoots, is involved in the production of proteins. A lack of this function in neurons can lead to serious neurological disorders such as disability and epilepsy.
Teams led by Marino Zerial from the Max Planck Institute (MPI) of Molecular Cell Biology and Genetics in Dresden and Stefan Raunser from the MPI of Molecular Physiology in Dortmund have made a significant discovery. Working in conjunction with colleagues from the MPI for Brain Research in Frankfurt am Main and the MPI for Biophysical Chemistry in Göttingen, they have identified a new mechanism that transports messenger RNA (mRNA), the blueprint for proteins, exactly where it’s needed within neurons.
Using an array of techniques, the researchers have identified a protein complex, named FERRY, which links mRNA to intra-cellular carriers, and elucidated its role and structure. The discovery may lead to a better understanding of neurological disorders caused by FERRY malfunction and possibly to new medical targets. The results are detailed in two recent works, published back-to-back in the journal Molecular Cell.

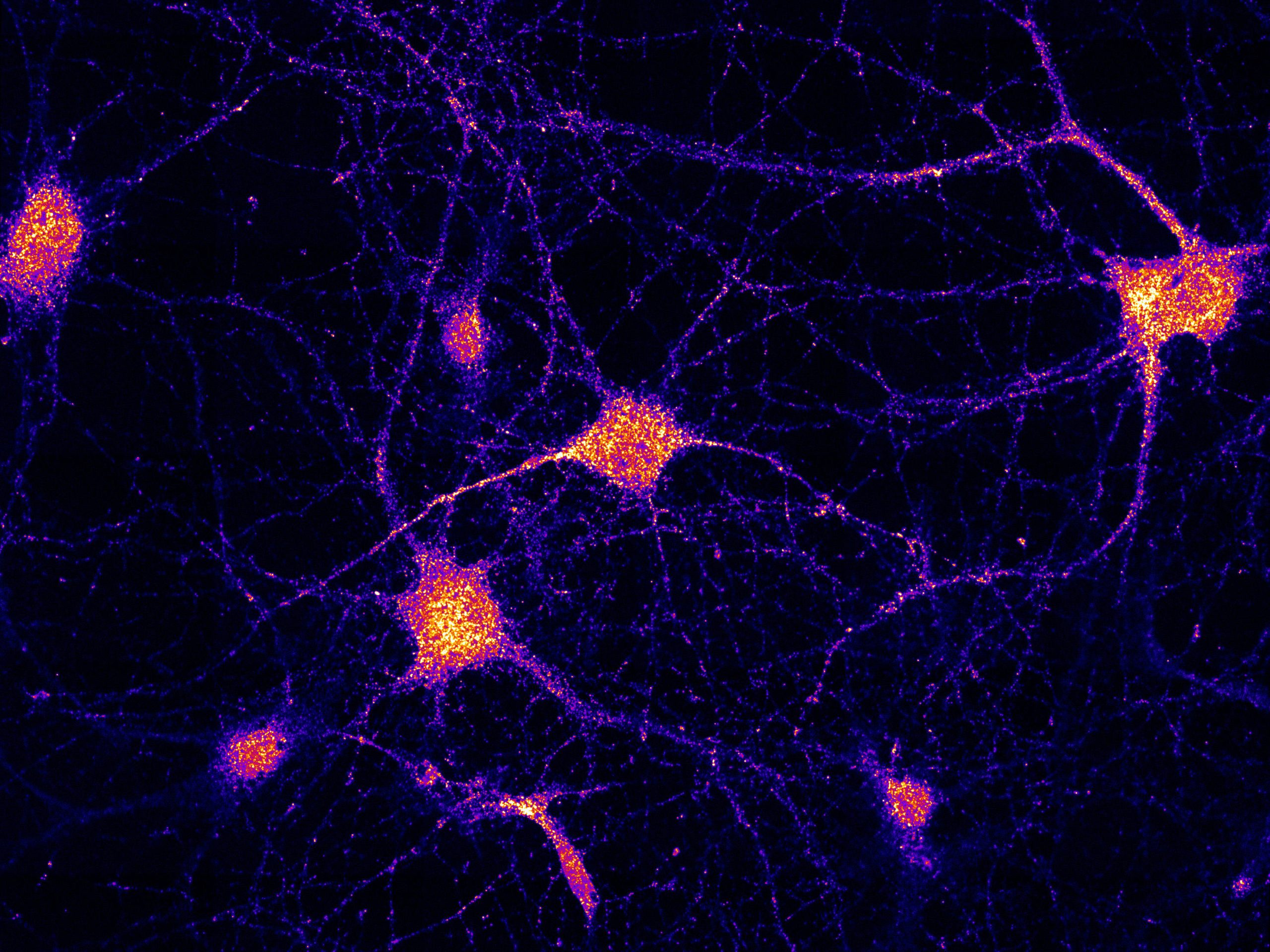
In neurons, FERRY is linked to EEs and works similarly to a tie-down strap during transport: It interacts directly with mRNA and holds it onto EEs, which hence become logistic carriers for mRNA transport and distribution in brain cells. Credit: Schuhmacher et al. (2023) / MPI-CBG
Faraway, so close!
“These publications provide a major advancement to elucidate the mechanisms underlying mRNA distribution in brain cells,” Marino Zerial says.
Cells produce vital proteins using mRNA as a blueprint and ribosomes as 3D printers. Yet, brain cells have a logistic challenge to overcome: A tree-like shape with branches that can span centimeters in the brain. “This implies that thousands of mRNAs need to be transported far away from the nucleus, resembling the logistic effort of properly supplying supermarkets in an entire country,” Jan Schuhmacher says, first author of the study.
So far, researchers attributed the carrier role to spherical compartments inside the cell, called Late Endosomes. However, MPI scientists argue that a different form of the compartments, called Early Endosomes (EEs), are also suitable as mRNA carriers, due to their ability to travel in both directions along intracellular road networks. In the first publication, led by Marino Zerial from MPI in Dresden, scientists discovered the function of a protein complex that they called FERRY (Five-subunit Endosomal Rab5 and RNA/ribosome intermediarY). In neurons, FERRY is linked to EEs and works similarly to a tie-down strap during transport: It interacts directly with mRNA and holds it onto EEs, which hence become logistic carriers for mRNA transport and distribution in brain cells.
Complex details
But how does FERRY bind to mRNA? That’s when Stefan Raunser’s group from the MPI Dortmund comes into play. In the second publication, Dennis Quentin et al. used cryo-electron microscopy (cryo-EM) to infer the structure of FERRY and the molecular features that allow the complex to bind to both EEs and mRNAs. The new 3D atomic model of FERRY, with a resolution of 4 Ångstroms, shows a novel mode of binding RNA, which involves coiled-coil domains. Scientists also explained how some genetic mutations affect FERRY’s ability to link mRNA thus leading to neurological disorders.
“Our research sets the groundwork for a more comprehensive understanding of neurological disorders caused by a failure of mRNA transport or distribution that might also lead to the identification of therapeutically relevant targets,” Raunser says.
References: “Structural basis of mRNA binding by the human FERRY Rab5 effector complex” by Dennis Quentin, Jan S. Schuhmacher, Björn U. Klink, Jeni Lauer, Tanvir R. Shaikh, Pim J. Huis in ’t Veld, Luisa M. Welp, Henning Urlaub, Marino Zerial and Stefan Raunser, 1 June 2023, Molecular Cell.
DOI: 10.1016/j.molcel.2023.05.009
“The Rab5 effector FERRY links early endosomes with mRNA localization” by Jan S. Schuhmacher, Susanne tom Dieck, Savvas Christoforidis, Cedric Landerer, Jimena Davila Gallesio, Lena Hersemann, Sarah Seifert, Ramona Schäfer, Angelika Giner, Agnes Toth-Petroczy, Yannis Kalaidzidis, Katherine E. Bohnsack, Markus T. Bohnsack, Erin M. Schuman and Marino Zerial, 1 June 2023, Molecular Cell.
DOI: 10.1016/j.molcel.2023.05.012





More Stories
Quelle est la prochaine grande nouveauté en matière de perte de poids ?
Une nouvelle découverte pourrait réécrire les livres sur la génétique
Compenser le sommeil le week-end pourrait réduire d’un cinquième le risque de maladie cardiaque – étude | Maladie cardiaque